Terrestrial Megafauna Response to Drone Noise Levels in Ex Situ Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Drone Noise Profile Recording
2.2. Species and Mammalian Baseline Audiogram
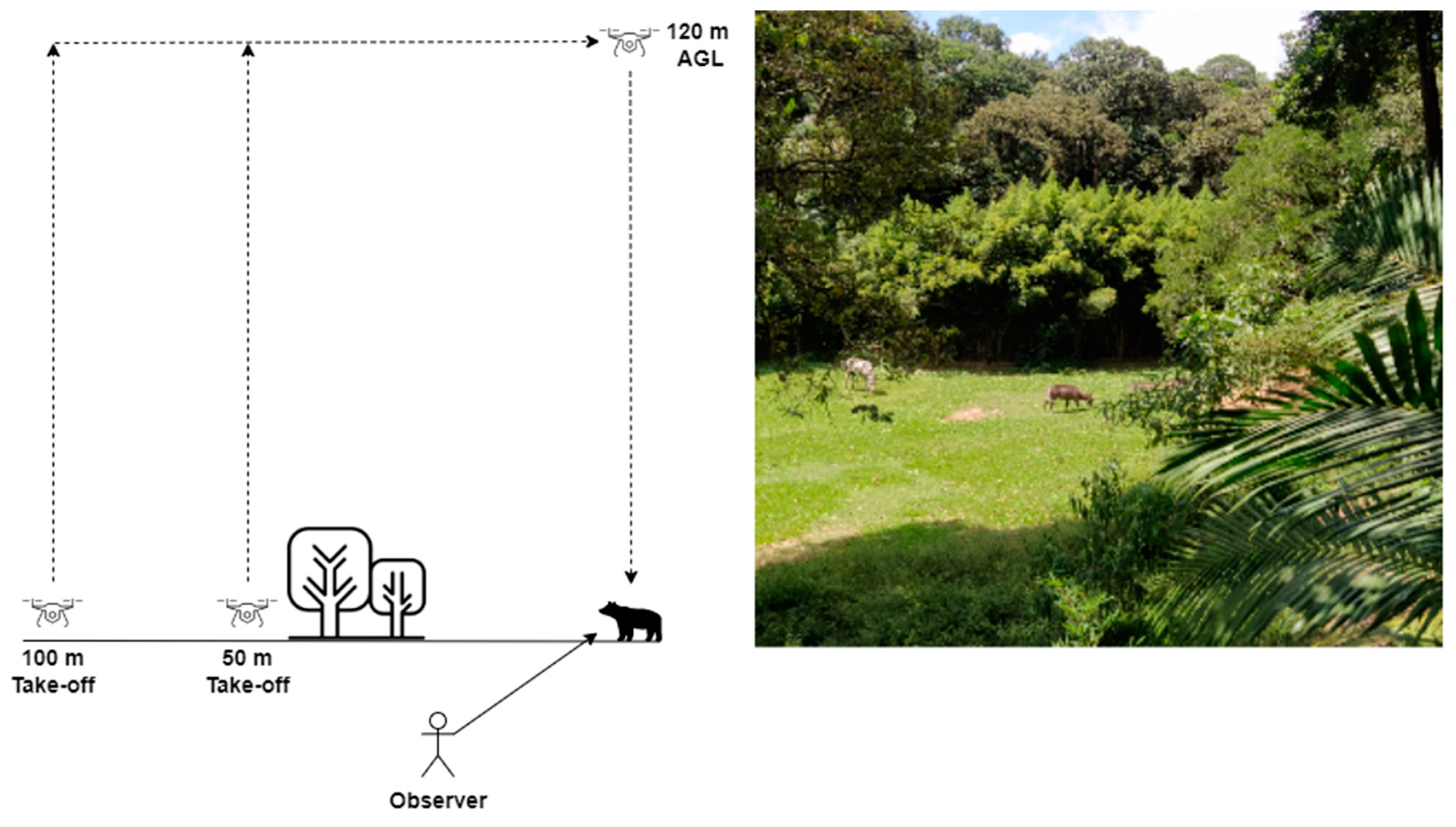
2.3. Recording Megafauna Behavior during Drone Exposure
2.4. Data Analysis
2.5. Ethical Note
3. Results
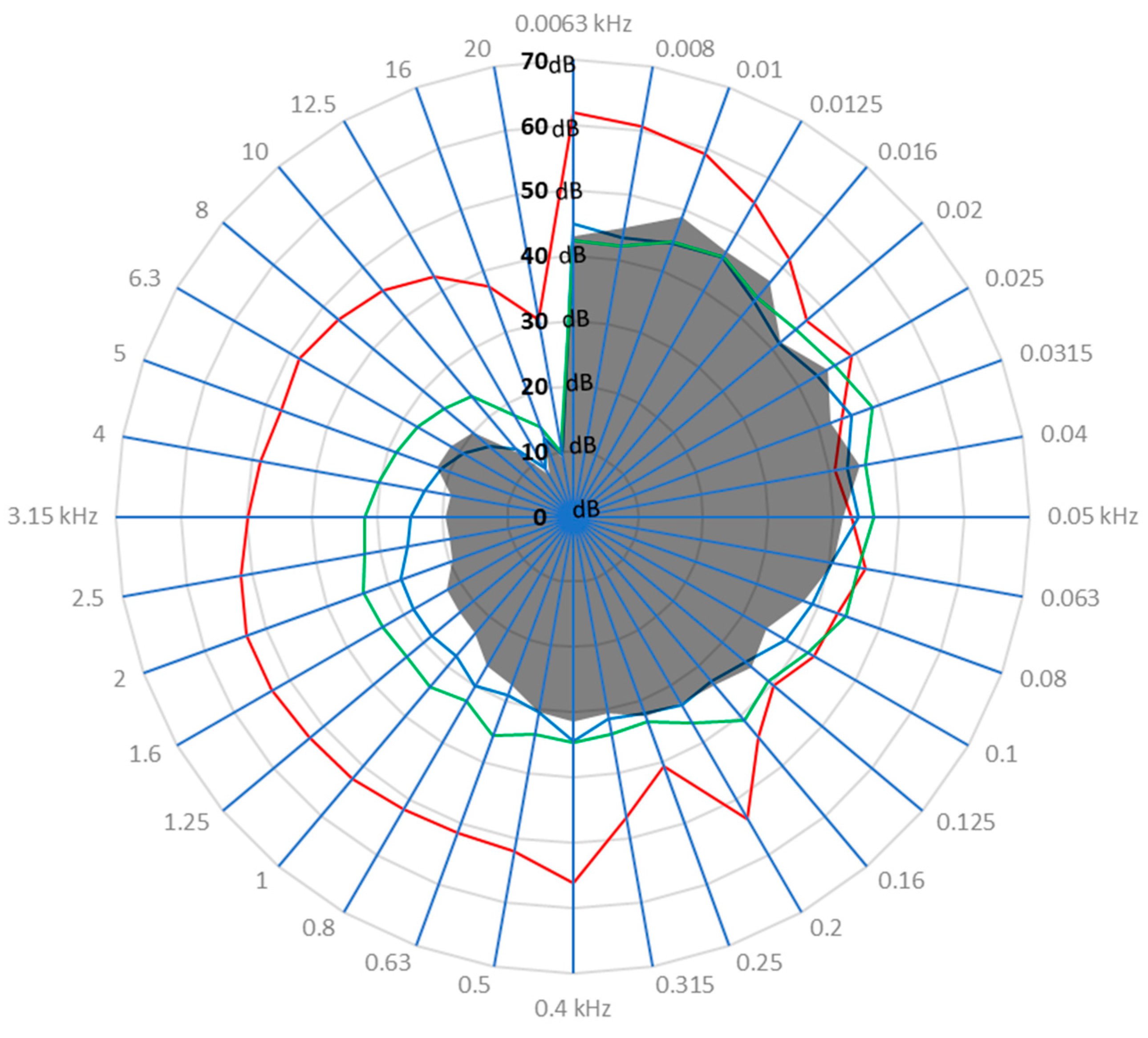
3.1. Drone Sound Profile
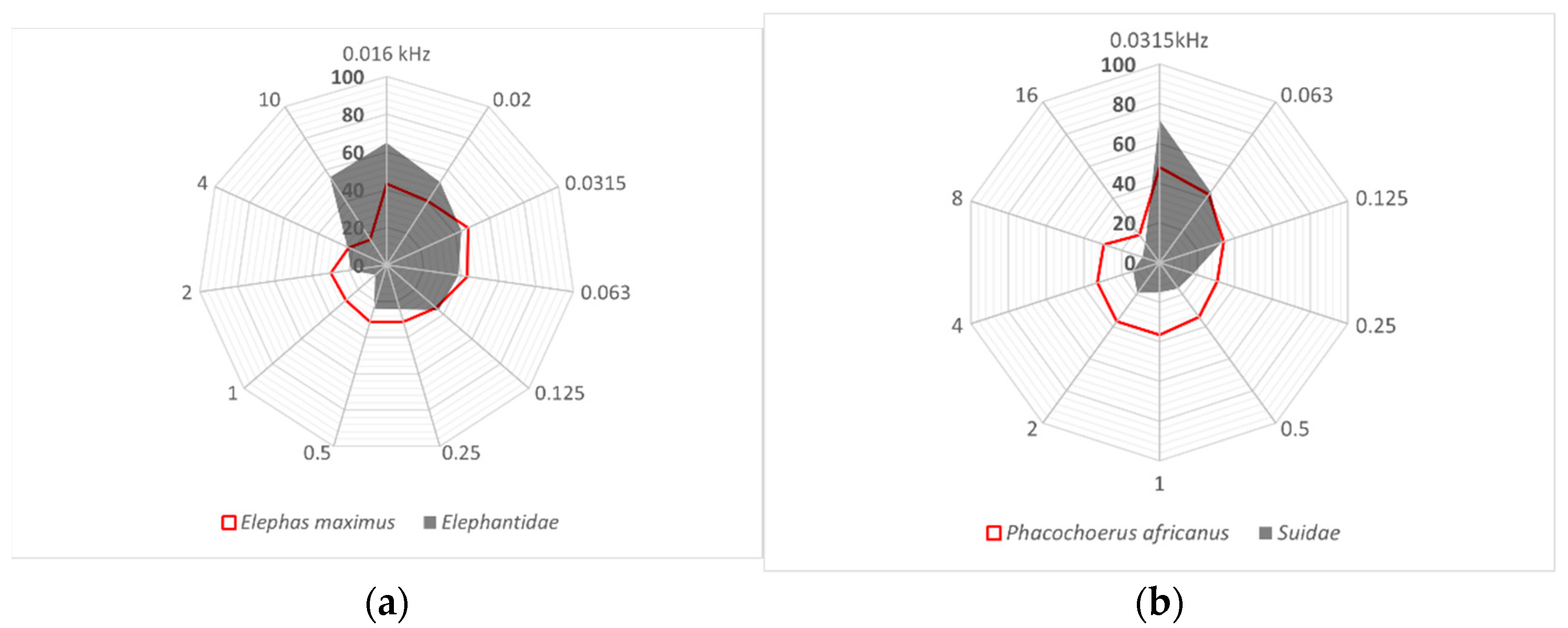
3.2. Limits of Hearing Sensitivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chabot, D.; Bird, D.M. Wildlife research and management methods in the 21st century: Where do unmanned aircraft fit in? J. Unmanned Veh. Syst. 2015, 3, 137–155. [Google Scholar] [CrossRef]
- Christie, K.S.; Gilbert, S.L.; Brown, C.L.; Hatfield, M.; Hanson, L. Unmanned aircraft systems in wildlife research: Current and future applications of a transformative technology. Front. Ecol. Environ. 2016, 14, 241–251. [Google Scholar] [CrossRef]
- Jiménez, L.J.; Mulero-Pázmány, M. Drones for Conservation in Protected Areas: Present and Future. Drones 2019, 3, 10. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UASs) the future of wildlife monitoring? A review of accomplishments and challenges. Mammal Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.T.; Wotherspoon, S.; Kilpatrick, A.D.; Raja Segaran, R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef]
- Kellenberger, B.; Marcos, D.; Tuia, D. Detecting mammals in UAV images: Best practices to address a substantially imbalanced dataset with deep learning. Remote Sens. Environ. 2018, 216, 139–153. [Google Scholar] [CrossRef]
- Rey, N.; Volpi, M.; Joost, S.; Tuia, D. Detecting animals in African Savanna with UAVs and the crowds. Remote Sens. Environ. 2017, 200, 341–351. [Google Scholar] [CrossRef]
- Schofield, G.; Esteban, N.; Katselidis, K.A.; Hays, G.C. Drones for research on sea turtles and other marine vertebrates—A review. Biol. Conserv. 2019, 238, 108214. [Google Scholar] [CrossRef]
- Schroeder, N.M.; Panebianco, A.; Gonzalez, M.R.; Carmanchahi, P. An experimental approach to evaluate the potential of drones in terrestrial mammal research: A gregarious ungulate as a study model. R. Soc. Open Sci. 2020, 7, 191482. [Google Scholar] [CrossRef]
- Bonnin, N.; Van Andel, A.; Kerby, J.; Piel, A.; Pintea, L.; Wich, S. Assessment of Chimpanzee Nest Detectability in Drone-Acquired Images. Drones 2018, 2, 17. [Google Scholar] [CrossRef]
- Olsoy, P.J.; Shipley, L.A.; Rachlow, J.L.; Forbey, J.S.; Glenn, N.F.; Burgess, M.A.; Thornton, D.H. Unmanned aerial systems measure structural habitat features for wildlife across multiple scales. Methods Ecol. Evol. 2018, 9, 594–604. [Google Scholar] [CrossRef]
- Mulero-Pázmány, M.; Jenni-eiermann, S.; Strebel, N.; Sattler, T.; Jose, J. Unmanned aircraft systems as a new source of disturbance for wildlife: A systematic review. PLoS ONE 2017, 12, e0178448. [Google Scholar] [CrossRef] [PubMed]
- Van der Vliet, R.E.; Jeninga, L.; van den Burg, A. RPAS over Natura 2000 areas: Disturbance responses of wildlife and opportunities for research RPAS over Natura 2000 areas: Disturbance responses of wildlife and opportunities for research. In Proceedings of the RPAS Civil Operators & Operations Forum 8th Annual International Conference, The Hague, The Netherlands, 30 December 2019. [Google Scholar]
- Brisson-Curadeau, É.; Bird, D.; Burke, C.; Fifield, D.A.; Pace, P.; Sherley, R.B.; Elliott, K.H. Seabird species vary in behavioural response to drone census. Sci. Rep. 2017, 7, 17884. [Google Scholar] [CrossRef]
- Mesquita, G.P.; Rodríguez-Teijeiro, J.D.; Wich, S.A.; Mulero-Pázmány, M. Measuring disturbance at swift breeding colonies due to the visual aspects of a drone: A quasi-experiment study. Curr. Zool. 2021, 67, 157–163. [Google Scholar] [CrossRef]
- Ditmer, M.A.; Vincent, J.B.; Werden, L.K.; Tanner, J.C.; Laske, T.G.; Iaizzo, P.A.; Garshelis, D.L.; Fieberg, J.R. Bears Show a Physiological but Limited Behavioral Response to Unmanned Aerial Vehicles. Curr. Biol. 2015, 25, 2278–2283. [Google Scholar] [CrossRef]
- Bennitt, E.; Bartlam-Brooks, H.L.A.; Hubel, T.Y.; Wilson, A.M. Terrestrial mammalian wildlife responses to Unmanned Aerial Systems approaches. Sci. Rep. 2019, 9, 2142. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Koh, L.P. Best practice for minimising unmanned aerial vehicle disturbance to wildlife in biological field research. Curr. Biol. 2016, 10, R404–R405. [Google Scholar] [CrossRef]
- Moleón, M.; Sánchez-Zapata, J.A.; Donázar, J.A.; Revilla, E.; Martín-López, B.; Gutiérrez-Cánovas, C.; Getz, W.M.; Morales-Reyes, Z.; Campos-Arceiz, A.; Crowder, L.B.; et al. Rethinking megafauna. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192643. [Google Scholar] [CrossRef]
- Rebolo-Ifrán, N.; Graña Grilli, M.; Lambertucci, S.A. Drones as a Threat to Wildlife: YouTube Complements Science in Providing Evidence about Their Effect. Environ. Conserv. 2019, 46, 205–210. [Google Scholar] [CrossRef]
- Scobie, C.A.; Hugenholtz, C.H. Wildlife monitoring with unmanned aerial vehicles: Quantifying distance to auditory detection. Wildl. Soc. Bull. 2016, 40, 781–785. [Google Scholar] [CrossRef]
- Duporge, I.; Spiegel, M.P.; Thomson, E.R.; Chapman, T.; Lamberth, C.; Pond, C.; Macdonald, D.W.; Wang, T.; Klinck, H. Determination of optimal flight altitude to minimise acoustic drone disturbance to wildlife using species audiograms. Methods Ecol. Evol. 2021, 12, 2196–2207. [Google Scholar] [CrossRef]
- Heffner, H.E.; Heffner, R.S. The Behavioral Study of Mammalian Hearing. In Springer Handbook of Auditory Research; Springer: Berlin/Heidelberg, Germany, 2014; pp. 269–285. [Google Scholar]
- Veilleux, C.C.; Kirk, E.C. Visual Acuity in Mammals: Effects of Eye Size and Ecology. Brain Behav. Evol. 2014, 83, 43–53. [Google Scholar] [CrossRef] [PubMed]
- ISO-3746; Acoustics—Determination of Sound Power Levels and Sound Energy Levels of Noise Sources Using Sound Pressure—Survey Method Using an Enveloping Measurement Surface over a Reflecting Plane. International Organization for Standardization: Geneva, Switzerland, 2010.
- ANAC—Agência Nacional de Aviação Civil. Regulamento Brasileiro de Aviação Civil Especial RBAC-E nº 94. Requisitos Gerais para Aeronaves Não Tripuladas de Uso Civil. Resolução nº 419. 2017. Available online: https://www.anac.gov.br/assuntos/legislacao/legislacao-1/rbha-e-rbac/rbac/rbac-e-94/@@display-file/arquivo_norma/RBACE94EMD00.pdf (accessed on 20 January 2021).
- Behavioral Audiograms of Mammals. Available online: https://www.utoledo.edu/al/psychology/research/psychobio/audiograms2.html (accessed on 15 July 2018).
- Da Silva Souza, G.; Gomes, B.D.; Silveira, L.C.L. Comparative neurophysiology of spatial luminance contrast sensitivity. Psychol. Neurosci. 2011, 4, 29–48. [Google Scholar] [CrossRef]
- Caves, E.M.; Brandley, N.C.; Johnsen, S. Visual Acuity and the Evolution of Signals. Trends Ecol. Evol. 2018, 33, 358–372. [Google Scholar] [CrossRef]
- Peixoto, N.H.; Ferreira, L.S. Higiene Ocupacional III; UFSM/CTISM: Santa Maria, Brazil; Rede e-Tec: Brasilia, Brazil, 2013. [Google Scholar]
- Sikes, R.S.; Gannon, W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Turner, J.G.; Bauer, C.A.; Rybak, L.P. Noise in animal facilities: Why it matters. J. Am. Assoc. Lab. Anim. Sci. 2007, 1, 10–13. [Google Scholar]
- Moss, C.J.; Croze, H.J. (Eds.) The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Chicago University Press: Chicago, IL, USA, 2008. [Google Scholar]
- Paula, R.C.; Gambarini, A. Histórias de um Lobo; Avis Brasilis Editora Vinhedo: São Paulo, Brazil, 2013; p. 264. [Google Scholar]
- Nowak, R.M. Walker’s Mammals of the World; The Johns Hopkins University Press: Baltimore, MD, USA; London, UK, 1999; p. 836. [Google Scholar]
- Sawyer, D.; Coutinho, B.; Figueiredo, I.; Navega, R. Perfil do Ecossistema Hotspot de Biodiversidade do Cerrado. 2017. Available online: http://cepfcerrado.iieb.org.br/wp-content/uploads/2017/09/PR_Cerrado-Perfildo-Ecossistema_TEXTOAPENDICES_port_revisada-20170804.compressed.pdf (accessed on 15 July 2018).
- Mulero-Pázmány, M.; Stolper, R.; van Essen, L.D.; Negro, J.J.; Sassen, T. Remotely Piloted Aircraft Systems as a Rhinoceros Anti-Poaching Tool in Africa. PLoS ONE 2014, 9, e83873. [Google Scholar] [CrossRef]
- ISO-R-226:2003; Normal Equal-Loudness Level Contours. International Organization for Standardization: Geneva, Switzerland, 2003. Available online: https://www.iso.org/standard/34222.html (accessed on 15 July 2018).
- Heffner, R.S.; Heffner, H.E. Hearing range of the domestic cat. Hear. Res. 1985, 19, 85–88. [Google Scholar] [CrossRef]
- Rosowski, J.J. Outer and Middle Ears. In Comparative Hearing: Mammals; Springer: Berlin/Heidelberg, Germany, 1994; pp. 172–247. [Google Scholar]
- Heffner, R.S.; Heffner, H.E. Hearing in the elephant (Elephas maximus): Absolute sensitivity, frequency discrimination, and sound localization. J. Comp. Physiol. Psychol. 1982, 96, 926–944. [Google Scholar] [CrossRef]
- Heffner, R.; Heffner, H. Hearing in large mammals: The horse (Equus caballus) and cattle (Bos taurus). Behav. Neurosci. 1983, 97, 299–309. [Google Scholar] [CrossRef]
- Heffner, H., Jr.; Heffner, H.E. The behavioral audiogram of whitetail deer (Odocoileus virginianus). J. Acoust. Soc. Am. 2010, 127, EL111–EL114. [Google Scholar] [CrossRef] [PubMed]
- Hosey, G.; Melfi, V.; Pankhurst, S. Zoo Animals: Behaviour, Management, and Welfare, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Barnas, A.F.; Felege, C.J.; Rockwell, R.F.; Ellis-Felege, S.N. A pilot(less) study on the use of an unmanned aircraft system for studying polar bears (Ursus maritimus). Polar Biol. 2018, 41, 1055–1062. [Google Scholar] [CrossRef]
- Mustafa, O.; Barbosa, A.; Krause, D.J.; Peter, H.-U.; Vieira, G.; Rümmler, M.-C. State of knowledge: Antarctic wildlife response to unmanned aerial systems. Polar Biol. 2018, 41, 2387–2398. [Google Scholar] [CrossRef]
- Braverman, I. Conservation without nature: The trouble with in situ versus ex situ conservation. Geoforum 2014, 51, 47–57. [Google Scholar] [CrossRef]

| Common Name | Species | Family |
|---|---|---|
| Addax | Addax nasomaculatus | Bovidae |
| Cattle | Bos taurus | Bovidae |
| Waterbuck | Kobus ellipsiprymnus | Bovidae |
| Dromedary | Camelus dromedarius | Camelidae |
| Maned wolf | Chrysocyon brachyurus | Canidae |
| Red deer | Cervus elaphus | Cervidae |
| Sambar | Rusa unicolor | Cervidae |
| Asian elephant | Elephas maximus | Elephantidae |
| Imperial zebra | Equus grevyi | Equidae |
| Jaguar | Panthera onca | Felidae |
| Bengal tiger | Panthera tigris tigris | Felidae |
| Giraffe | Giraffa camelopardalis | Giraffidae |
| Hippopotamus | Hippopotamus amphibius | Hippopotamidae |
| Giant anteater | Myrmecophaga tridactyla | Myrmecophagidae |
| White rhinoceros | Ceratotherium simum simum | Rhinocerotidae |
| Warthog | Phacochoerus africanus | Suidae |
| Tapir | Tapirus terrestris | Tapiridae |
| Spectacled bear | Tremarctos ornatus | Ursidae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesquita, G.P.; Mulero-Pázmány, M.; Wich, S.A.; Rodríguez-Teijeiro, J.D. Terrestrial Megafauna Response to Drone Noise Levels in Ex Situ Areas. Drones 2022, 6, 333. https://doi.org/10.3390/drones6110333
Mesquita GP, Mulero-Pázmány M, Wich SA, Rodríguez-Teijeiro JD. Terrestrial Megafauna Response to Drone Noise Levels in Ex Situ Areas. Drones. 2022; 6(11):333. https://doi.org/10.3390/drones6110333
Chicago/Turabian StyleMesquita, Geison Pires, Margarita Mulero-Pázmány, Serge A. Wich, and José Domingo Rodríguez-Teijeiro. 2022. "Terrestrial Megafauna Response to Drone Noise Levels in Ex Situ Areas" Drones 6, no. 11: 333. https://doi.org/10.3390/drones6110333
APA StyleMesquita, G. P., Mulero-Pázmány, M., Wich, S. A., & Rodríguez-Teijeiro, J. D. (2022). Terrestrial Megafauna Response to Drone Noise Levels in Ex Situ Areas. Drones, 6(11), 333. https://doi.org/10.3390/drones6110333








